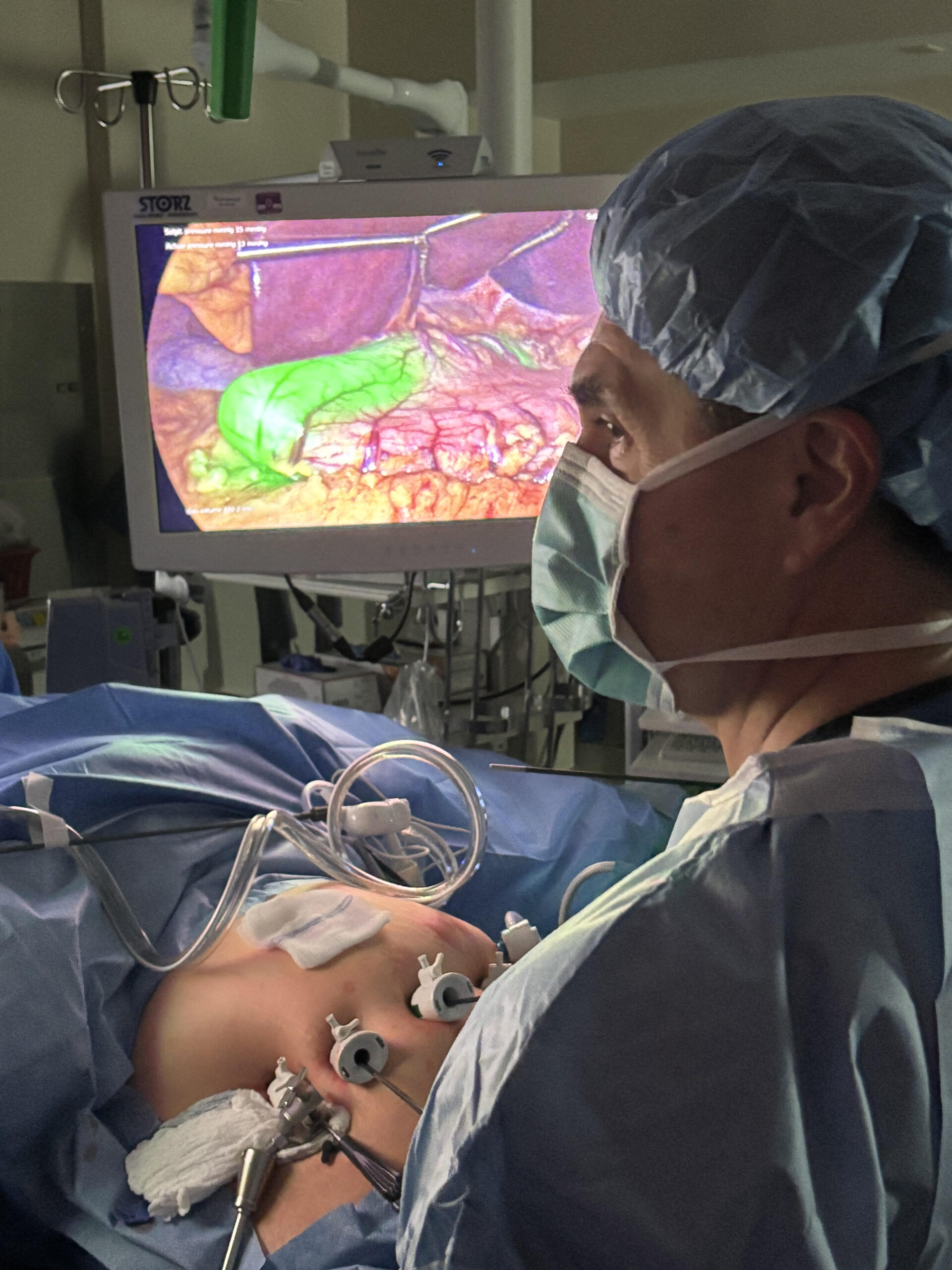
Endolumik’s Latest Patent Fuels Innovation in Surgery, Validated by Leading Surgeons. See how advancements in fluorescence-guided technology—praised by Surgeons like Dr. Jaime Ponce—are transforming robotic and laparoscopic procedures in our latest press release! https://www.pr.com/press-release/927695
Category: Article
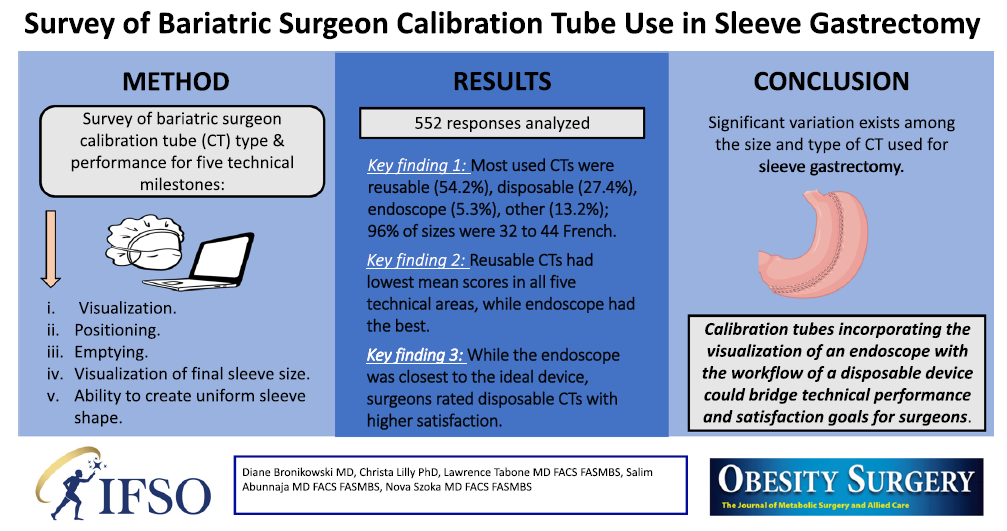
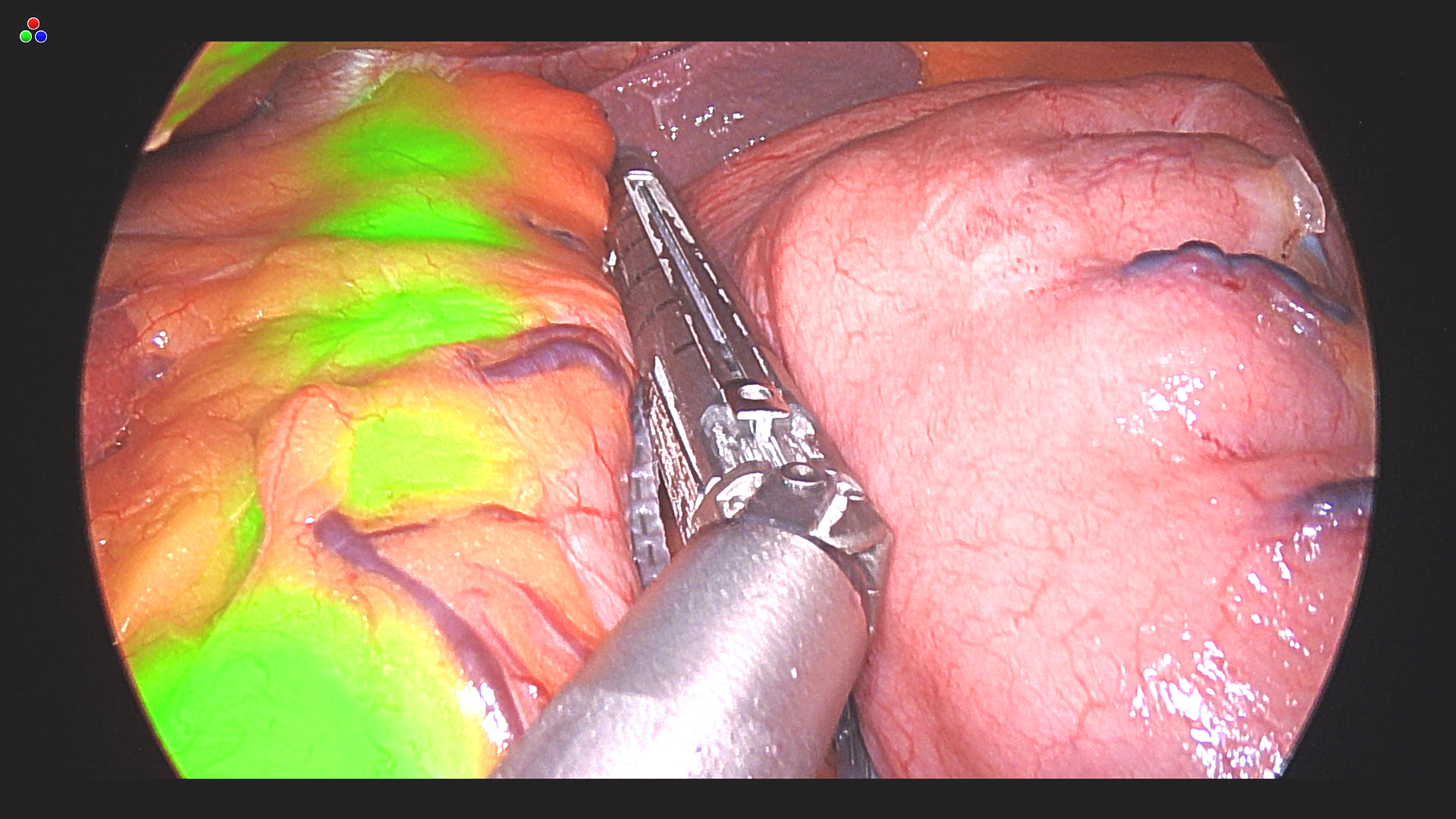
📢 Exciting research highlights the variability in calibration tube performance during sleeve gastrectomy. At Endolumik, we’re bridging the gap between surgeon satisfaction and technical precision with innovative solutions.
🔑 Key takeaways from the study:
•Significant variation exists with regards to size/type of calibration tube used for sleeve gastrectomy
•While surgeons favored visualization and performance seen with the endoscope, disposable calibration tubes had the highest satisfaction for streamlined workflows
Endolumik’s technology is designed to address these very challenges, offering optimal visualization and technical performance to elevate surgical outcomes. Let’s shape the future of bariatric surgery together! 💡
Click on the image below to view the study:
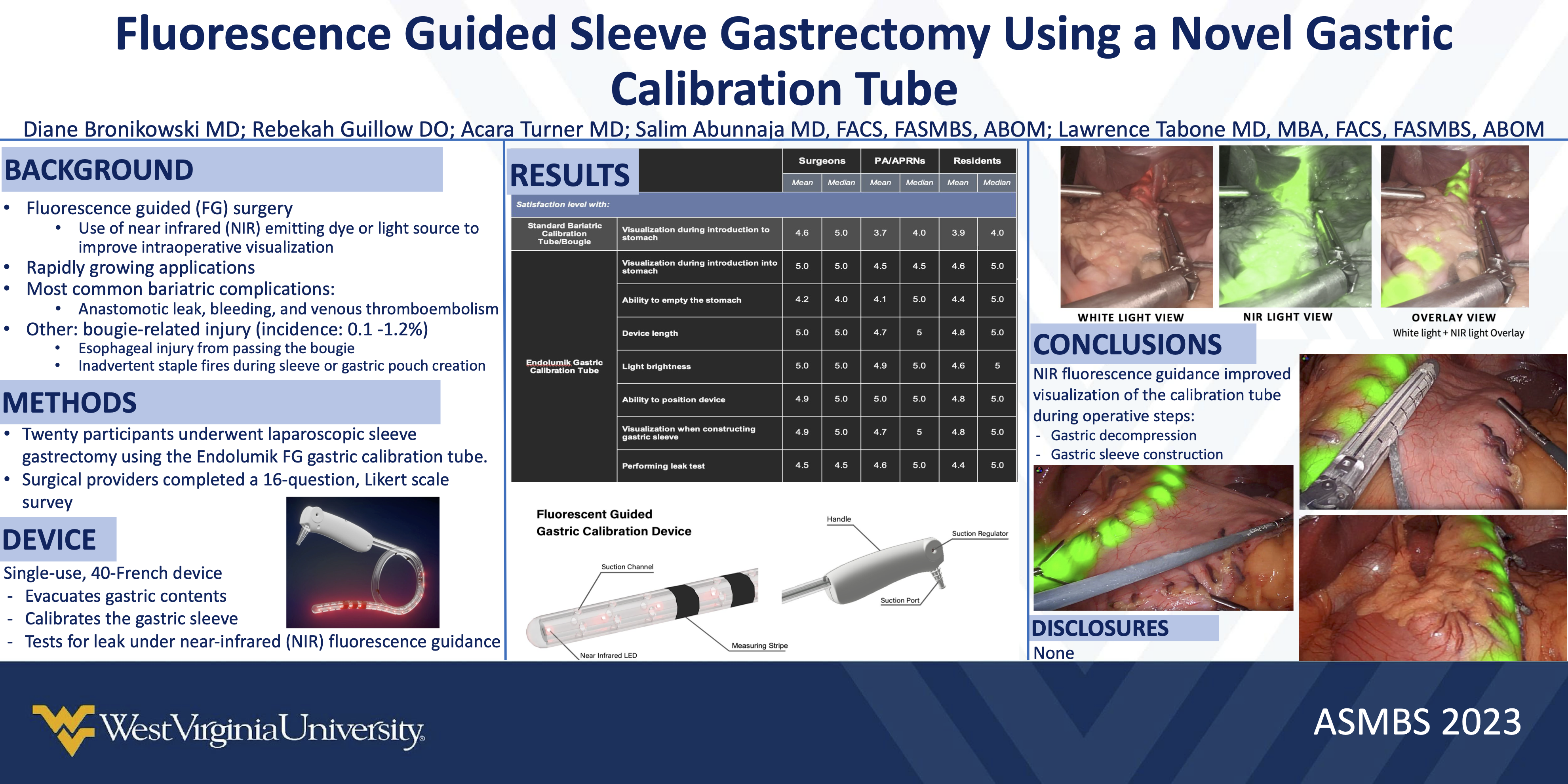
Endolumik is thrilled to be exhibiting at ASMBS 2024 this year! Check out our ePoster from 2023’s ASMBS Annual Meeting as a sneak peak for this year. 
Did you have the chance to explore Endolumik’s ePoster showcased at SAGES 2021? Don’t miss out—check it out now!
Thank you so much to editors Sean Whooley, Jim Hammerand, Chris Newmarker, and Danielle Kirsh for recognizing the transformative and first ever FDA Safer Technology Program device, the Endolumik Gastric Calibration Tube! Read the article below:
https://www.medicaltubingandextrusion.com/catheter-based-innovations-we-cant-stop-thinking-about/
We are thrilled to be featured as one of the 124 novel devices granted marketing authorization by the CDRH in 2023. To read the full report, check out the link below:
Want to see the Endolumik Gastric Calibration Tube in action? Head over to Endolumik’s Youtube page to check out our latest videos 📺🎬
Check out Dr. Salim Abunnaja‘s captivating presentation, linked below, at The 26th World Congress of the IFSO International Federation for The Surgery of Obesity and Metabolic Disorders held on September 1, 2023 in Naples, Italy. Fantastic insight on Endolumik’s gastric calibration tube!
Congratulations to Nova Szoka MD, FACS, FASMBS, co-founder of Endolumik, along with David Renton and Santiago Horgan, for the release of The SAGES Manual of Fluorescence-Guided Surgery! This textbook offers an in-depth and state-of-the-art perspective on FGS across various surgical disciplines, written by specialists who excel in utilizing FGS in their respective surgical areas. We are very proud to be founded by an innovative and respected thought leader like Nova, and to be on the cutting edge of this new generation of surgical tools! Check out the textbook linked below.

Farmington, NM, November 27, 2023 –(PR.com)– Endolumik, an innovator in minimally-invasive surgical devices, announced another breakthrough procedure. Dr. Philip Ernest and Dr. James Boyd, General and Bariatric Surgeons at San Juan Regional Medical Center, performed the first ever fluorescent guided bariatric surgery in the state of New Mexico, using the company’s new Endolumik Gastric Calibration Tube.
Endolumik’s innovative surgical tool recently received 510(k) authorization as the first device ever through the FDA’s Safer TEchnology Program, or STEP. According to the FDA, the STEP program is for devices that are “reasonably expected to significantly improve the safety of currently available treatments.” The NIR fluorescence of the Endolumik device is designed to help surgeons avoid adverse events that can be caused by poor visualization. This will help make it the safest calibration tube available.
“At San Juan Regional Medical Center, our commitment is to seamlessly integrate cutting-edge technology with surgical procedures to enhance safety and overall outcomes. Notably, Endolumik stands out as the sole FDA-approved sizing instrument for bariatric surgery recognized as a safety device,” said Dr. Ernest, Director of the Metabolic and Bariatric Institute at San Juan Regional Medical Center. “Incorporating Endolumik into our robotic bariatric surgeries represents a significant advancement in patient safety, contributing to excellent results. We are enthusiastic about adopting a product that aligns with the overarching goals of our surgical program. This integration of advanced technology underscores our dedication to providing the highest standard of care to our patients.”
The device was invented by another bariatric surgeon, Dr. Nova Szoka, FACS, FASMBS, specifically to improve safety and performance in these procedures. The company is excited to be using the technology to help bring safe procedures to populations that often don’t have access to quality metabolic care. Endolumik CEO Mara McFadden notes, “We are proud to be partnering with innovators like San Juan Regional Medical Center to help give access to the best technology to as many patients as possible.”
San Juan Regional Medical Center recently launched its Metabolic and Bariatric Institute to help bring much needed bariatric surgery services to a traditionally underserved patient population. The Institute accepted its first patients this summer providing both surgical and medical options to help patients achieve their weight loss goals and improve their overall health and wellness.
Endolumik is a medical device company developing novel tools for laparoscopic surgery. Its patented fluorescence guided surgical tools are designed to help make minimally invasive surgical procedures safer and more effective. Learn more at www.endolumik.com.
San Juan Regional Medical Center is a non-profit, acute care hospital and Level III Trauma Center with 198 licensed beds. Better is our mission, improving lives through personalized health and care. As a sole community provider in San Juan County, we deliver a remarkable range of highly personalized and specialized healthcare services to the people of the entire Four Corners region. San Juan Regional Medical Center is accredited by DNV. More information is available at sanjuanregional.com.
Omaha, NE, June 09, 2023 –(PR.com)– Endolumik, an innovator in minimally-invasive surgical devices, today announced that its fluorescence guided Gastric Calibration Tube has begun commercial sales, starting with the prestigious Nebraska Medicine Bariatrics Center.
Endolumik’s novel surgical tool has been used extensively in clinical trials and recently received 510(k) authorization as the first device ever through the FDA’s Safer TEchnology Program, or STEP. According to the FDA, the STEP program is for devices that are “reasonably expected to significantly improve the safety of currently available treatments.” The NIR fluorescence of the Endolumik device is designed to help surgeons avoid adverse events that can be caused by poor visualization. This procedure with UNMC will mark the first introduction of the device for public use.
Dr. Corrigan McBride, DABOM, FACS, FASMBS, and the Chief of General Surgery, Minimally Invasive Surgery and Bariatrics at UNMC, was eager to be a leader with the device. She is not only the Director of the MBSAQIP accredited Bariatrics Center, but is also a leading researcher in laparoscopic metabolic surgery. “Innovation is one of the core values of Nebraska Medicine and UNMC, so when I saw the Enolumik I was excited about the opportunity to improve patient safety through a new surgical device”
The Endolumik Gastric Calibration Tube uses near infrared (NIR) light to allow surgeons to more clearly visualize surgical tools inside the stomach. The inventor of the device, Dr. Nova Szoka, FACS, FASMBS notes, “It’s our goal to provide surgeons with additional visual cues to help them improve their performance, and standardize surgical outcomes for patients.”
The event also marks an interesting moment of progress in the traditionally male-dominated surgical field. Endolumik is a women-owned, women-led company, founded on the invention of a female surgeon. When Dr. McBride expressed her interest to be one of the first users, both parties thought it seemed a fitting opportunity to have a woman surgeon lead the way for this technological innovation. Dr. David W. Mercer, the Chair of Surgery at UNMC, “Dr. McBride is a leader in Minimally Invasive and Bariatric Surgery. She is always working to improve safety and outcomes for her patients.”
Endolumik is launching the device with a select number of leading hospitals and surgery centers throughout the spring. “We are looking for partners who not only appreciate the technological value of this device for patients, but are excited to be ushering in a new era of higher performing digital surgical tools,” said Endolumik CEO Mara McFadden.